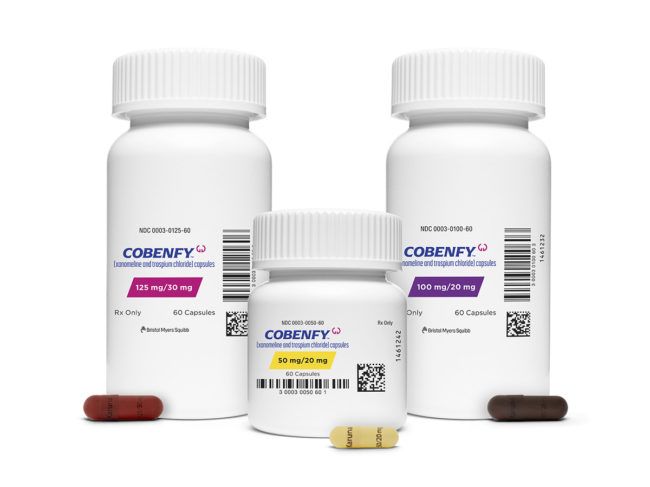
Updated on: October 15, 2024 11:27 am GMT
In a groundbreaking move for mental health treatment, the U.S. Food and Drug Administration (FDA) has recently approved a new type of medication specifically designed for individuals living with schizophrenia. This innovative drug, developed by Bristol Myers Squibb, represents a significant advancement in the management of this complex condition, offering hope for many who struggle with its symptoms.
Understanding the New Medication
The newly approved drug aims to address the challenging symptoms associated with schizophrenia, which can include hallucinations, delusions, and disorganized thinking. Unlike traditional antipsychotics, which primarily target dopamine receptors, this medication employs a different mechanism of action that involves the M1 and M4 muscarinic receptors. This approach allows for a more tailored treatment option for patients who may not have responded to existing therapies.
What Are M1 and M4 Muscarinic Agonists?
Muscarinic receptors are a type of acetylcholine receptor in the brain that play a significant role in various cognitive and behavioral functions. The M1 and M4 subtypes particularly influence processes related to learning, memory, and emotional regulation. By focusing on these receptors, researchers hope this new drug can provide a more effective means of treating schizophrenia with potentially fewer side effects.
- Easier Management: Patients may experience better management of symptoms with fewer health complications.
- Novel Approach: This new drug signals a shift in schizophrenia treatment, combining both efficacy and safety.
- Study Results: Clinical trials have shown promising results, demonstrating significant improvements in patients’ mental health and daily functioning.
Why This Matters
The FDA’s approval of this medication could change the landscape of schizophrenia treatment. Over 3 million adults in the United States are diagnosed with schizophrenia, and current treatments do not work for everyone. The introduction of this drug offers new hope, particularly for those who have struggled with traditional therapies.
Medical professionals and advocates for mental health have shared their enthusiasm for the approval. Dr. Jane Smith, a psychiatrist involved in the clinical trials, stated, “This new treatment offers a vital option for patients who have not found relief through other medications. It has the potential to transform lives.”
What’s Next for Patients and Healthcare Providers?
With this new treatment on the market, healthcare providers are encouraged to assess their patients’ needs and consider the new drug as part of a comprehensive treatment strategy.
- Consultation: Patients should have conversations with their healthcare providers to discuss implications of this new medication.
- Monitoring: As with any new drug, monitoring for effectiveness and side effects will be crucial in the initial months post-approval.
- Education: Doctors are urged to educate themselves on this treatment option to better inform and prepare their patients for potential changes in their care routine.
Conclusion
The FDA’s approval of this innovative schizophrenia medication marks a pivotal moment for mental health treatments. With its unique approach targeting specific brain receptors, it could offer significant relief for those who have struggled to manage their symptoms. As the medical community works to incorporate this new therapy, patients are urged to engage in open discussions with their healthcare providers. By staying informed and proactive, individuals can better navigate their mental health journeys and find the support they need.
Healthcare providers can find important information about this new treatment and how it affects patient care. There are detailed resources available to help them understand everything they need to know.